Team finds most complex protein knot ever seen
An MIT team has discovered the most complicated knot ever seen in a protein, and they believe it may be linked to the protein's function as a rescue agent for proteins marked for destruction.
"In proteins, the three-dimensional structure is very important to the function, and this is just one example," said Peter Virnau, a postdoctoral fellow in physics and an author of a paper on the work that appears in the Sept. 15 issue of the Public Library of Science, Computational Biology.
Knots are rare in proteins - less than 1 percent of all proteins have any knots, and most are fairly simple. The researchers analyzed 32,853 proteins, using a computational technique never before applied to proteins at this scale.
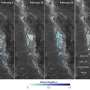
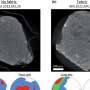
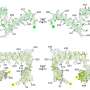
Of those that had knots, all were enzymes. Most had a simple three-crossing, or trefoil knot, a few had four crossings, and the most complicated, a five-crossing knot, was initially found in only one protein - ubiquitin hydrolase.
That complex knot may hold some protective value for ubiquitin hydrolase, whose function is to rescue other proteins from being destroyed - a dangerous job.
When a protein in a cell needs to be destroyed, it gets labeled with another protein called ubiquitin. "It's a death mark for the protein," said Leonid Mirny, an author of the paper and an associate professor in the MIT-Harvard Division of Health Sciences and Technology.
Once the "death mark" is applied, proteins are shuttled to a cell structure called a proteasome, which pulls the protein in and chops it into pieces. However, if ubiquitin hydrolase intervenes and removes the ubiquitin, the protein is saved.
The complicated knot found in ubiquitin hydrolase may prevent it from getting sucked into the proteasome as it works, Mirny said. The researchers hypothesize that proteins with complex knots can't be pulled into the proteasome as easily, and the knots may make it harder for the protein to unfold, which is necessary for degradation.
The same knot is found in ubiquitin hydrolase in humans and in yeast, supporting the theory that there is a connection between the knot and the protein's function. This also seems to suggest that the knot has been "highly preserved throughout evolution," Virnau said.
Until now, scientists have not paid much attention to knots in proteins, but the MIT researchers hope their work will ignite further interest in the subject. "We just hope this will become a part of the routine crystallographers and NMR spectroscopists do when they solve a structure," Mirny said.
Virnau is working on a computer program and a web server, soon to be publicly available, that can analyze the structure of any protein to see if it has knots, which he believes could be helpful to researchers in structural genomics. (Structural genomics aims to determine the structure of all proteins produced by a given organism.)
Since their initial screening, the researchers have discovered five-crossing knots in two other proteins - a brain protein whose overexpression and mutations are linked with cancer and Parkinson's disease, and a protein involved in the HIV replication cycle.
They have also found examples of proteins that are closely related and structurally similar except for the presence or absence of a knot. Two versions of the enzyme transcarbamylase, from humans and certain bacteria, catalyze different reactions, depending on whether or not there is a knot. The researchers speculate that somewhere along the evolutionary line, the sequence that allowed a protein to form the knot was added or deleted.
The third author on the paper is Mehran Kardar, an MIT physics professor. The research was funded by the National Science Foundation and the German Research Foundation.
Source: MIT