Discovering How to Focus on Tiniest of the Very Small
If you need a good picture of a molecule, your first job is getting its atoms to pose for you, says John Silcox, Cornell's David E. Burr Professor of Engineering and an expert in the realm of the very tiny.
But atoms are not willing subjects. They jiggle furiously, defying any microscopist who tries to catch them at a standstill. Nor are they polite: The larger atoms in a molecule typically overshadow the smaller ones, making it impossible to view the little ones.
Now, though, researchers at Cornell have developed a technique to get a closer-than-ever look at individual atoms within crystal molecules -- allowing them, for the first time, to see the polarity, or physical alignment, of those constituent atoms and to get a view of the smaller atoms.
The research -- by Cornell postdoctoral associate K. Andre Mkhoyan, Silcox and colleagues at Cornell, and Philip Batson of IBM -- is described in the June 2 issue of Science.
With the new technique, researchers can better predict the physical properties of a crystal at every point -- an advance that offers potential improvements in lasers and other devices, particularly at the nanoscale, where the structure of an individual molecule can determine a device's behavior.
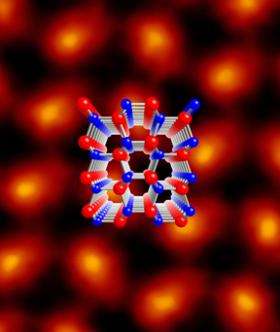
To get their new and improved view, Mkhoyan's team used a scanning transmission electron microscope (STEM) at IBM on samples of aluminum nitride, gallium nitride and other crystals with particular significance in nanotechnology research, in a chamber padded and shielded to reduce potentially atom-jiggling acoustic noise and electromagnetic radiation. Fitting the STEM with an aberration corrector (a focusing device) developed at Nion Co., they directed a 0.9 angstroms-wide electron beam at tiny crystal samples, collecting the scattered electrons on a ring-shaped detector and forming an image based on the resulting scatter pattern. (An angstrom is one hundred-millionth of a centimeter). Because larger atoms deflect electrons at a larger angle than small ones, the resulting data is relatively simple to interpret.
Used on a sample of aluminum nitride, the technique, called annular dark imaging, shows pear-shaped molecule columns with the larger aluminum atoms at the thicker end and the smaller nitrogen atoms at the narrower end. It is the first time the smaller atoms in such a structure have been caught in an image.
The key, said Silcox, is the narrowness of the scanning electron beam.
"We're down to the atom size, as opposed to the atom spacing," said Silcox. "We can start to see the light atom columns; we can characterize the crystal very nicely and precisely, at every place on the structure."
Mkhoyan said the inability to capture such images in the past has been a huge hurdle for nanotechnology researchers.
"The study and application of these lattice crystals are at the core of nanotechnology. Many papers are dedicated to synthesis and application of the nanoparticles -- quantum dots, rods, wires, you name it -- based on these materials," he said. "However, the performance of the devices is highly dependent on the structural quality of these nanoparticles.
"With our STEM annular dark field imaging, we come to the rescue," Mkhoyan added. "We can zoom in, pick up any region of the structure, and see how it behaves."
Source: Cornell University