Scientists identify two new forms of ice
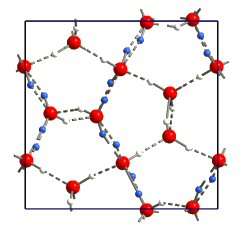
Scientists have discovered two previously unknown forms of ice, frozen at temperatures of around minus 160 degrees Celsius. The researchers say they have solved the atomic structures of the new forms, which they have named ice XIII and XIV.
The results, just published in the journal Science, could help improve our understanding of the role of water in life-supporting processes. Furthermore, the research team believes the new ices may also occur on the icy moons of the outer planets.
A team from Oxford University in collaboration with the CCLRC (Council for the Central Laboratory of the Research Councils), and UCL (University College London) carried out the research. For a long time scientists have known about twelve different ice forms, but this finding confirms what many have suspected, that other ices exist at much lower temperatures.
Dr Christoph Salzmann, from the Chemistry department of Oxford University, said: 'We knew from theory that these ice forms should exist. The problem, however, was the low temperatures at which they were expected to form. We therefore had to search for a catalyst, which would enhance molecular mobility at low temperatures and encourage the phases to form.'
Professor Paolo Radaelli, from CCLRC, said: 'The key to this discovery was to persuade water molecules to order fully – something they are usually very reluctant to do.'
Dr Salzmann's method was uncannily simple - just a few drops of hydrochloric acid and some carefully applied pressure did the trick. They were then able to measure the temperatures of formation, which were at around minus 160 degrees Celsius, and a beam from the powerful neutron instruments at ISIS, the world's leading pulsed neutron facility in Oxfordshire, revealed the atomic structures.
Professor John Finney, from UCL, who was also a member of the team that discovered the previous ice form, ice XII, said: 'Scientists have been searching for such a formation recipe for over forty years. Having now found it, the way looks open to finally completing our knowledge of the crystalline behaviour of this amazing - yet critically important - simple molecule.'
This discovery gives scientists a better knowledge of the hydrogen bond in the many crystalline forms of ice. It also improves the current understanding of the water molecule in chemical and biological processes, and could enhance computational models used in chemistry and structural biology.
Understanding the behaviour of water at low temperatures provides greater insight into the state and behaviour of water in the solar system. High-pressure low temperature ice forms are thought to be present on the outer planets possibly as the result of meteoroid impacts on their surfaces.
Source: University of Oxford