Revising Earth's early history
Earth's future was determined at birth. Using refined techniques to study rocks, researchers at the Carnegie Institution's Department of Terrestrial Magnetism found that Earth's mantle--the layer between the core and the crust--separated into chemically distinct layers faster and earlier than previously believed. The layering happened within 30 million years of the solar system's formation, instead of occurring gradually over more than 4 billion years, as the standard model suggests. The new work was recognized by Science magazine, in its December 23 issue, as one of the science breakthroughs for 2005.
Carnegie scientists Maud Boyet and Richard Carlson analyzed isotopes--atoms of an element with the same number of protons, but a different number of neutrons--of elements in rock samples for their work. As Carlson explains, "Isotopes exist naturally in different proportions and are used to determine conditions under which rock forms. Radioactive isotopes are particularly handy because they decay at a predictable rate and can reveal a sample's age and when its chemical composition was established."
In the standard model of the geochemical evolution of the Earth, the Earth's mantle has been evolving gradually over Earth's 4.567-billion-year history primarily through the formation of the chemically distinct continental crust. Shortly after solid material began condensing from the hot gas of the cooling early solar system, the object that would become Earth grew by the collision and accretion of smaller rocky bodies. The chemical composition of these building blocks is preserved today in primitive meteorites called chondrites.
In the 1980s, scientists analyzed the ratio of isotopes of the rare earth element neodymium in chondrites and various terrestrial rocks collected at or near the Earth's surface and found that the samples shared a common composition. Researchers believed that this ratio remained constant from the beginning of Earth formation. Using new-generation equipment, Boyet and Carlson found, surprisingly, that the terrestrial samples did not have the same ratio as the meteorites. Compared to chondrites, all terrestrial rocks measured have an excess of the mass 142 isotope of neodymium (142Nd), which is the decay product of a now-extinct radioactive isotope of samarium of mass 146 (146Sm) that was present at the birth of the solar system but decayed away shortly thereafter. The excess in 142Nd allowed the researchers to determine when the composition of the Earth diverged from that of the meteorites--within the first 30 million years after solar system formation, which is less than 1% of the age of our planet.
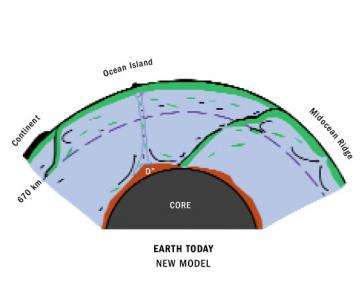
To explain the excess of 142Nd found in the terrestrial samples, the Carnegie scientists believe that the Earth was largely molten during its formation and that rapid crystallization of Earth's early magma ocean caused the mantle to separate into chemically distinct layers, one containing a high ratio of Sm to Nd similar to that observed today in the mantle source of the volcanism along ocean ridges. The complementary reservoir, with low 142Nd abundance, has never been sampled at the surface and hence could now be deeply buried in the so-called D" layer at the very base of the mantle, above the core. This "missing" layer should be rich in the elements uranium, thorium, and potassium, whose long-lived radioactive decay heats Earth's interior and causes our planet to remain geologically active. This hot layer above the core could help to keep the outer core molten so that circulation of liquid iron can produce Earth's magnetic field, and it could instigate the hot plumes of upwelling mantle material that give rise to volcanically active islands, such as Hawaii.
Measurements by Boyet and Carlson also show that lunar rocks have the same abundance of 142Nd as the terrestrial samples, a finding that adds to the evidence that the Moon formed from the Earth. Since Mars also experienced early melting, as indicated by the chemical and isotopic composition of Martian meteorites, the new results now link the early evolution of Earth, Moon, and Mars and highlights the importance of early events in determining the chemical characteristics of the terrestrial planets.
"The work of Boyet and Carlson, when added to what has already been determined for the Moon and Mars, shows that the earliest days of the inner planets were violent times in solar system history," adds DTM director Sean Solomon. "Theoretical work by Carnegie scientist George Wetherill had pointed to this result, but now we have a clear chemical signature of this episode of Earth history."
Source: Carnegie Institution