Nottingham research sheds new light on how chemical reactions work
Research from The University of Nottingham’s School of Chemistry has contributed to a breakthrough in the complex world of understanding how the quantum mechanics of chemical reactions work.
By understanding chemical processes better chemists will be able to conduct experiments more quickly and accurately, and make new chemicals more cheaply and efficiently.
A study led by Dr Stuart Althorpe, Reader in Physical Chemistry, is published in the August 19 issue of the prestigious international journal Science.
The research was carried out as part of a long-standing collaboration with a colleague at the University of Durham, Dr Eckart Wrede, and provides a leap forward for scientists all over the world.
Dr Althorpe said: “This work provides another vital piece of the jigsaw for understanding how chemical reactions work.
“Since the late 1920s chemists have been trying to gain a better understanding of all the different factors that occur during a chemical reaction particularly in terms of quantum mechanics — or put simply, how atoms and molecules behave during a chemical reaction. Our research takes us an important step closer to fully understanding these chemical processes in the greatest possible detail.”
Dr Wrede added: “This research will be helpful to solve reactions which can cause pollution in combustion processes or in the atmosphere.
“It can help to narrow down which reactions are the most polluting and should be examined more urgently to find ways to reduce their effects.”
The Nottingham group used a sophisticated supercomputer, the £5m High Performance Computing (HPC) facility, to calculate the quantum behaviour of the atoms and molecules throughout a chemical reaction. The HPC, which had its official launch at the University Park campus earlier this year, is one of the world’s most powerful supercomputers and can perform three million million calculations per second.
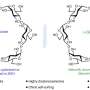
The Durham group then created a ‘billiard ball movie’ which allowed them to watch the motion of the atoms and molecules and learn more about how they reacted with each other. They found that only in certain situations did the movement of atoms and molecules speed up or slow down a chemical reaction.
Professor David Clary, of Oxford University, has written a Science Perspectives article on the research in the same issue of Science. He said: "The clever paper by Dr Althorpe and co-workers is a novel and definitive theoretical study on the simplest chemical reaction of hydrogen atoms with hydrogen molecules."
Source: University of Nottingham