Scientists discover the origin of a mysterious force
Scientists at the Universitat Autònoma de Barcelona and Imperial College London have discovered the origin of hydration force, a phenomenon that causes some complex chemical and biochemical species (including DNA and other electrostatically charged molecules) to repel at short distances when surrounded by water. Through this research, improvements could be made to the design of chemical products used in the chemical, pharmaceutical and food industry.
Ever since the 1970s, scientists have been trying to establish the cause of a repulsive force occurring between different electrostatically charged molecules, such as DNA and other biomolecules, when they are very close to each other in aqueous media. This force became know as hydration force.
Jordi Faraudo, a researcher for the Department of Physics at the Universitat Autònoma de Barcelona, and Fernando Bresme of the Department of Chemistry at Imperial College London have studied this mysterious force in detail and have discovered where its origins lie.
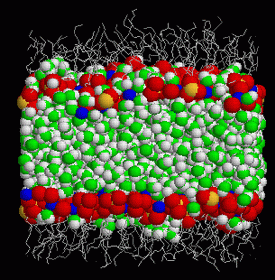
In the same way that a flag flutters in the direction the wind is blowing, at a microscopic level water molecules are gently attracted towards the direction in which an electric field is pointing. However, when the water is in contact with surfaces that create small electric fields, such as chemical compounds like those found in many detergents, this is no longer the case: the water molecules have a remarkable capacity to organise themselves into complex structures that are strongly orientated in such a way as to cancel out the electric field, and on some occasions, to reverse it. This abnormal behaviour was discovered by the same researchers and published in Physical Review Letters in April 2004.
The scientists have now discovered that this strange property is responsible for the hydration force that acts when water is surrounded by certain types of electrostatically charged molecules, such as DNA and some biological compounds, and when thin films form in detergents. The discovery has been published in today’s edition of Physical Review Letters.
Water is the solvent in which most physical, chemical and biological processes take place. Therefore, it is essential to understand the nature of interactions between molecules dissolved in water in order to understand many of these processes. Two of the most important of these processes are the adherence of substances to cell membranes and the withdrawal of proteins. Both of these are fundamental in biomedical research, since a substantial part of the process of designing new drugs is based on understanding how substances penetrate cell membranes to enter cells. These drugs are often proteins designed to prevent or strengthen the action of other substances. In these cases, accurately identifying the protein folding is essential, since the form these proteins take on when they fold influences how effectively they are able to act.
Fully understanding the properties of this force that occurs when molecules surrounded by water adhere to each other is also useful in the chemical industry, particularly when involving mechanisms in which colloidal suspensions must be stabilised, such as the mechanisms used to produce paints, cosmetics and food products such as yoghurt and mayonnaise.
Source: Universitat Autònoma de Barcelona